BACKGROUND:
One of the important properties of carbon is its tetravalency. Carbon is a strict octet follower, which means it needs a maximum of 8 electrons to form stable compounds. Since a carbon atom has 4 valence electrons, it can form up to 4 bonds with different elements. Part of the reason why there are millions of compounds of carbon is its possibility of forming very stable bonds with another carbon atom.
A carbon atom in an organic compound is labeled or classified based on the number of bonds and type of atoms attached to it. In this post, we will specifically focus on the classification of carbon based on the number of other carbon atoms attached to it.
In this case, C3 is an example of a tertiary carbon. Using the classification above, we will know that C5 and C6 are primary carbons while C4 is a secondary carbon. This classification scheme is important as it also applies in classifying organic compounds. Carbon content in tons C per ton coal equivalent = 0.746 ± 2% (The 0.746 value includes a heating value adjustment to recognize that the carbon content, developed on a higher heating value basis,must be increased when used with UN production data based on'net' or lower heating values.).
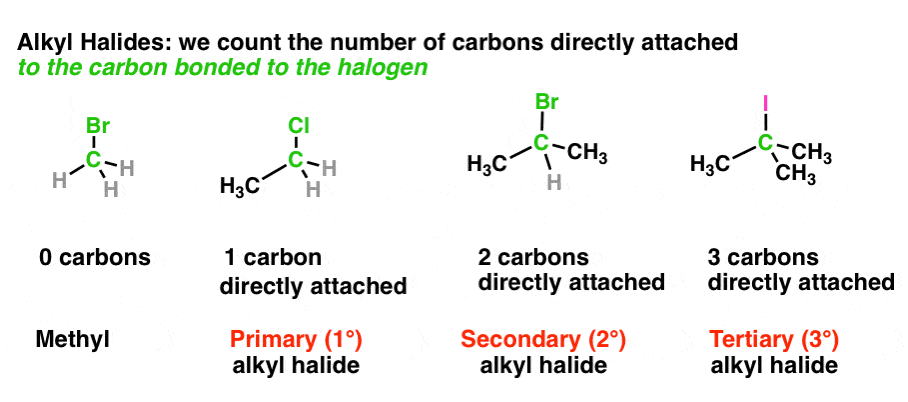
Carbon can be classified as primary, secondary, tertiary or quaternary depending on the number of carbon atoms it is bonded to. This classification only applies to saturated carbons. The classifications are as follow:
- Primary Carbon (1°) – Carbon attached to one other carbon
- Secondary Carbon (2°) – Carbon attached to two other carbons
- Tertiary Carbon (3°) – Carbon attached to three other carbons
3-methylpentane
If we look at the example above of 3-methylpentane, C 1 is attached to three hydrogen atoms and only one carbon atom. This means based on the classification described above, C1 is a primary carbon. On the other hand, C2 is connected to only 2 hydrogen atoms and 2 other carbon atoms. In this case, C2 is considered a secondary carbon. Lastly, C3 is connected to only one hydrogen atom and three other carbon atoms. In this case, C3 is an example of a tertiary carbon. Using the classification above, we will know that C5 and C6 are primary carbons while C4 is a secondary carbon.
This classification scheme is important as it also applies in classifying organic compounds with different functional groups. This specifically applies for alcohols, amines, and alkyl halides. It also applies in classifying carbocations and carbanions.
Note: Each vertex in the condensed formula above is a carbon atom. Since the compound does not have multiple bonds, each carbon atom will have the maximum number it can have less the number of other carbon atoms directly attached to them.
MODELLING:
To demonstrate this topic, we’ll model 2-methylbutane using our Duluth Labs Molecular Sets which has the structure shown below:
2-methylbutane, also called isopentane, is a branched-chain alkane containing 5 carbon atoms. This compound is volatile and flammable and is commonly used in the close loops of geothermal powerplants to drive turbines.
Part I -Primary Carbon
1
1. Get a carbon atom (black) and connect three Hydrogen atoms (white) using shortmedium connectors
2
2. Attach another carbon atom using a medium
Note: Since the 1st Carbon only has one other carbon atom attached to it, then the 1st Carbon is considered a Primary carbon.
Part II - Secondary Carbon
1. To the structure you prepared in Part I, attach two hydrogen atoms to Carbon 2 using short medium connectors. Then, Attach another carbon atom to Carbon 2 using a medium connector and also attach a hydrogen..
Note: Since the 1nd Carbon has two other carbon atoms attached to it, then the 2nd Carbon is considered a Secondary carbon.
Part III - Tertiary Carbon
1. Attach two more carbon atoms to Carbon 3 using medium connectors
Note: Since the 3rd Carbon has three other carbon atoms attached to it, then the 3rd Carbon is considered a Tertiary carbon.
Complete the structure by connecting two hydrogen atoms each to the added carbon atoms using smallmedium connectors.
PRACTICE EXERCISE
How many primary, secondary, and tertiary carbons are present in the 1-sec-butyl-2-methylcyclopentane?
ANSWERS TO EXERCISES
There are four secondary carbon atoms, 3 primary carbon atoms, and 3 tertiary carbon atoms.
Exam 1 Answer Key
Professor Carl C. Wamser
Exam 1 Answer Key | Professor Carl C. Wamser |
1. (15 points) Write complete names for each of the following.
a)
trans-1-isopropyl-3-(2,2-dimethylpropyl)cyclopentane
b)
3-ethyl-2,2,5-trimethyl-7-(1-methylcyclohexyl)nonane
c)
2-bromo-1,3-dichloro-2,5-dimethylhexane
2. (15 points) Write accurate structures for the following:
a) a Newman diagram of gauche pentane
b) a compound of formula C5H12 with one tertiary carbon atom
c) 1-ethyl-5-methylspiro[2.4]heptane
d) the best chair conformation of trans-1,3-dimethylcyclohexane

e) the best Lewis structure for HCNO (bonded in that order)
3. (10 points) Complete the structures on the right so they are identical to the structures on the left.
The atom with the heavy dot should be the same one in both images.
a)
b)
4. (15 points) Complete each of the following acid-base reactions. Identify the pKa values of the acids on each side of the equation and predict the preferred direction of the equilibrium.
a)
b)
c)
d)
Of all the compounds cited above (on both sides of the equations), which is the strongest acid?
Which is the strongest base?
Which equation will have the most balanced equilibrium constant (closest to [products] = [reactants])?
(b) - the pKa values are the closest together
5. (15 points) Arrange the following in order with respect to the property indicated. Write MOST and LEAST under the compounds with the highest and lowest values of that property.
a) acidity
b) boiling point
c) stability
d) heat of combustion per mole
e) fraction of equatorial Cl at equilibrium
6. (10 points) Bicyclo[3.1.1]heptane has a little bit of conformational mobility.Write its structure with a good chair conformation in two ways, illustrating the chair interconversion.
Tertiary Carbon
Place a methyl group at C3 on both conformations and predict which would be preferred.
7. (20 points) Write Newman diagrams in all three staggered conformations for 1,2-dibromobutane.
(HINT - first write the line structure so you start out right)
Primary Carbon Secondary Tertiary
looking down the C1-C2 bond -
looking down the C2-C3 bond -
Assuming the relative group sizes are in the following order, predict the relative stabilities of each structure within each group of three conformations.
Tertiary Carbon E2
Br > CH2Br > CH2CH3 > CH3 > H
